UDI Services
Our UDI Services
We provide comprehensive support throughout the UDI lifecycle — from strategy and DI/PI creation to GUDID registration and FDA audit preparation.
What is UDI?
The Unique Device Identification (UDI) system is a regulatory requirement established by the U.S. Food and Drug Administration (FDA) to improve the identification and traceability of medical devices throughout their distribution and use. It enhances patient safety, facilitates recalls, and supports better post-market surveillance.
UDI Components:
A complete UDI consists of two key elements:
Device Identifier (DI): A fixed portion that identifies the specific version or model of a device and its manufacturer.
Production Identifier (PI): A variable portion that may include the device’s lot number, serial number, expiration date, and/or manufacturing date.
Regulatory Requirements:
Assign a UDI to each device and packaging level.
Submit device information to the FDA Global Unique Device Identification Database (GUDID).
Ensure UDI is present in both human-readable and AIDC (Automatic Identification and Data Capture) formats on device labeling.
Maintain compliance with UDI requirements according to device class and applicable timelines.
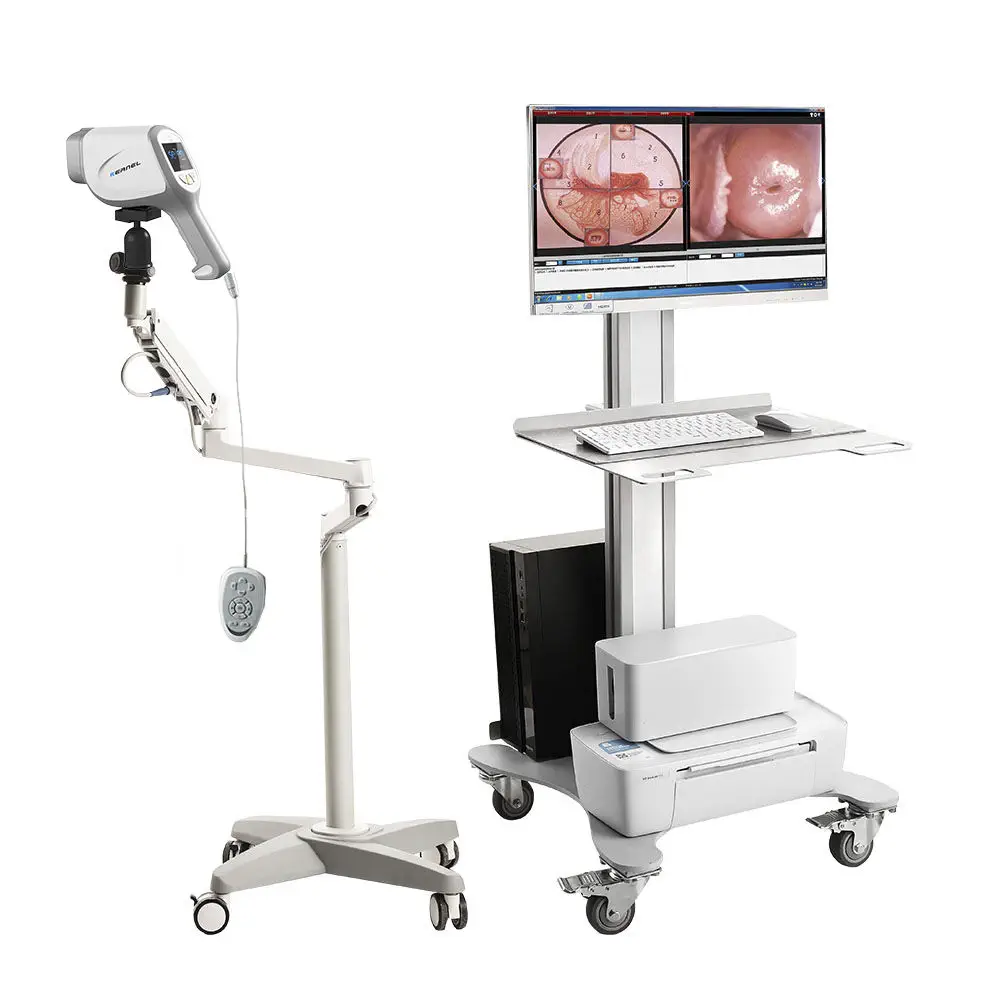
Medical Device Experience
We have hands-on expertise with a wide range of medical devices, ensuring precise UDI implementation and compliance. Our experience includes:
- Microscopes – General & Plastic Surgery
- Colposcopes – Obstetrics / Gynecology
- Light Sources – Gastroenterology / Urology
- Surgical Cameras – General & Plastic Surgery
- Rigid & Flexible Endoscopes – Ear, Nose & Throat
- Biopsy Needles – General Surgery
- Laryngostroboscopes – Ear, Nose & Throat
- Headlights – Ophthalmic


How AYM Consulting Services Can Help:
AYM Consulting Services LLC offers expert support in:
Get Your Free Consultation
Reach Us
Location :
Based in Overland Park, Kansas – Serving clients across the U.S. and Latin America
